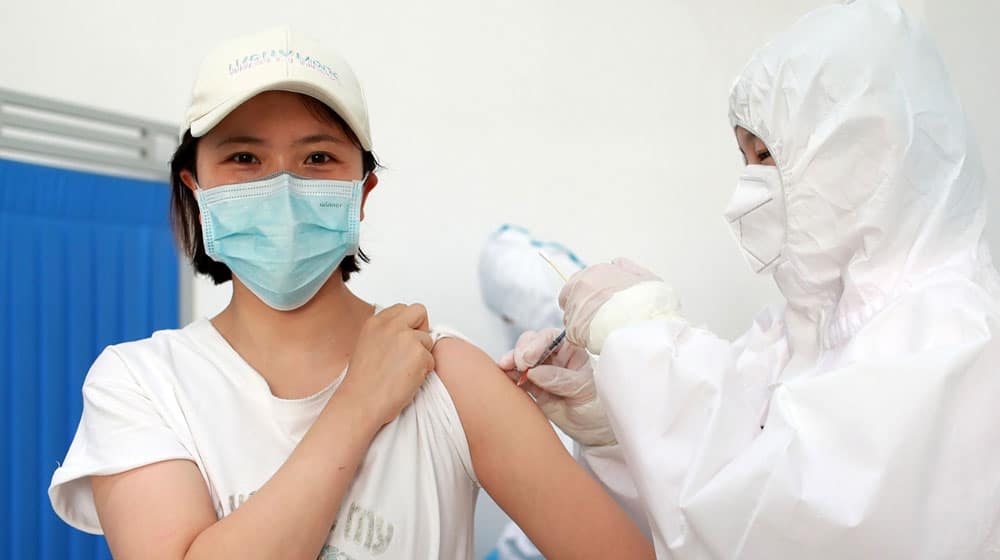
Coronavirus vaccine trials being held at the University of Health Sciences (UHS) are expected to conclude this week as 17,000 out of 18,000 volunteers have been administered the vaccine.
According to Vice-Chancellor UHS, Prof. Dr. Javed Akram, the vaccine has triggered the intended immune response among the trials’ participants, adding that detailed findings of the clinical trials will be published soon as well.
ALSO READ
PM Imran Officially Inaugurates the Special Technology Zones Authority
Dr. Akram noted that the process to register the Chinese vaccine with the Drug Regulatory Authority of Pakistan (DRAP) is well underway, adding that the vaccine would cost around $3-4.
He said that the chain of transmission of COVID-19 disease would break once 75% of the Pakistani population get vaccinated against the viral infection.
ALSO READ
KE Gets License to Construct and Operate RLNG Pipeline
The candidate vaccine, known as Ad5-nCoV, has been developed by CanSinoBIO, a Chinese vaccine manufacturer, in collaboration with the Beijing Institute of Biotechnology.
In August last year, DRAP granted permission to UHS to organize the clinical trials of CanSinoBIO’s COVID-19 vaccine. After making the necessary arrangements, UHS started the trial in September.

























ABE KIYA SARE MULK K VACINE PAKISTAN MAIN HE TRY HONGE ?
JISKO DEKHO APNA TRAIL YAHI KARWA RAHA HAI
TOMORROW I AM GOING TO TAKE PART IN THESE TRIALS AS HUMANITY. LETS SEE
Good job Sir.