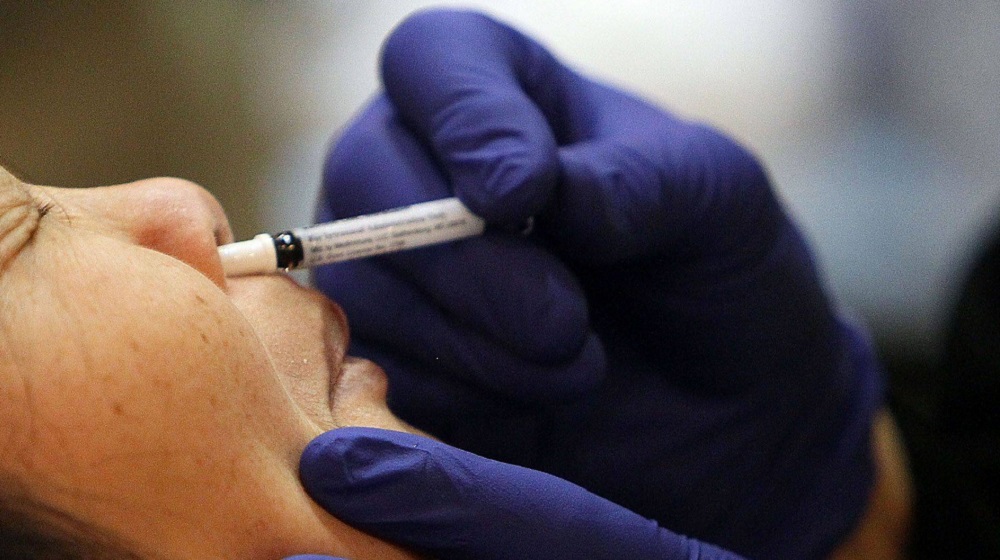
University of Health Sciences (UHS) has made arrangements to hold clinical trials for a nasal vaccine for COVID-19 developed by a Chinese firm.
According to details, the clinical trials will begin in six to eight weeks subject to approval from three committees.
ALSO READ
Pakistanis Break Records for Eid Cash Withdrawals at ATMs
While addressing the media about the development, Vice-Chancellor UHS, Dr. Javed Akram, said the first approval will be required from the UHS’s review board followed by second approval from the National Bioethics Committee. Drug Regulatory Authority of Pakistan (DRAP) will give the final approval to UHS for conducting clinical trials of the nasal COVID-19 vaccine.
He added the nasal COVID-19 vaccine is a single dose and has been manufactured by the CanSino Biologics whose single-dose intravenous vaccine is already being used in Pakistan.
ALSO READ
Shahid Afridi Shares a Heartbreaking News for PSL Fans
Around 5,000 volunteers will take part in the clinical trials at the UHS to determine the efficacy and safety of the nasal COVID-19 vaccine, noted Dr. Akram, adding that volunteers would be tested through PCR tests and next-generation sequencing to analyze the effectiveness of the vaccine.
Dr. Akram expressed hope that the vaccine will generate the intended immune response at the site of the infection and within the respiratory passage as the vaccine is already being used in China.